Analysis of electrodialysis as a method of producing potable water
(1) Palos Verdes High School
https://doi.org/10.59720/23-010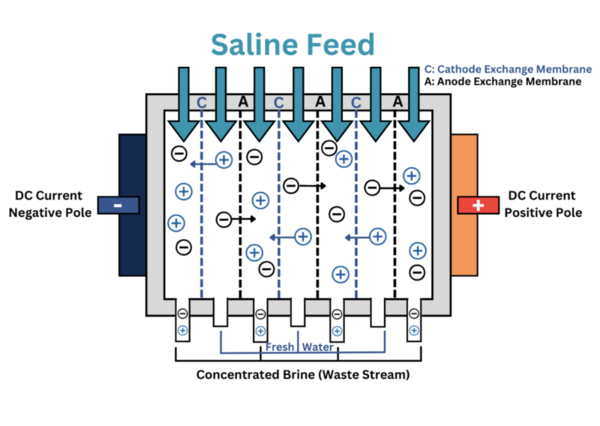
Only 1 percent of the water on Earth is available for human consumption and usage, and scientists predict that this severe shortage will affect the entire planet by 2050. To resolve this problem, we collected samples of ionized seawater from along the coasts of the San Pedro Channel and Santa Monica Bay and measured their respective total dissolved solids (TDS) every hour as it underwent electrodialysis until the measurement was under the acceptable TDS for drinking water. Our objective was to produce large amounts of purified water through the separation process of electrodialysis, which if successful, would be advantageous to improving the water shortage crisis. Our hypothesis was that after saltwater undergoes electrodialysis, the time it takes for the Na+ cations to pass through the cathode exchange membranes and Cl- anions to pass through the anode exchange membranes will decrease as the voltage from the Direct Current (DC) power circuit is increased, which in turn increases the electric current. Our experimentation showed that the final recordings of the deionized water’s TDS were under the acceptable TDS for consumable water for all experiment trials. The results of this experiment may be beneficial in increasing the percentage of water available for human consumption.
This article has been tagged with: