Peptidomimetics Targeting the Polo-box Domain of Polo-like Kinase 1
(1) Poolesville High School, Poolesville, Maryland, (2) Chemical Biology Laboratory, National Cancer Institute at Frederick, Frederick, Maryland
https://doi.org/10.59720/16-032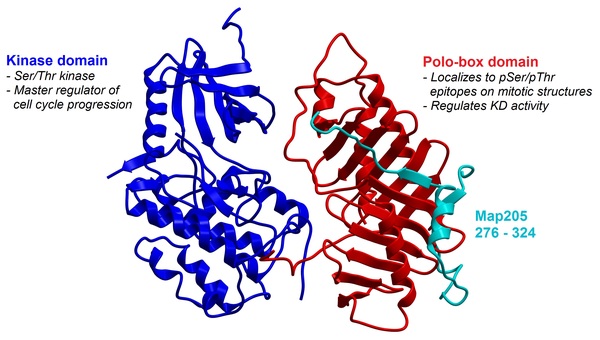
Polo-like kinase 1 (Plk1) is a master regulator of mitosis, activating key proteins that are involved in processes such as spindle assembly, centrosome maturation, and anaphase regulation. Certain types of cancers, including breast and stomach cancers, rely heavily on Plk1 overexpression for their growth and proliferation. Therefore, Plk1 is being investigated as a target for cancer drugs. Efforts to inhibit Plk1 have focused on the development of ATP-competitive small molecules directed at the kinase domain; however, these compounds can have dose-limiting toxicities attributed to off-target inhibition. Alternatively, peptide-based ligands targeted to the C-terminal polo-box domain (PBD) can inhibit the proper localization of Plk1, resulting in disrupted kinase activity and mitotic arrest. Here we synthesized and evaluated peptides and peptidomimetics derived from proteins known to interact with Plk1, which include polo-box interacting protein 1 (PBIP1), 205-kDa microtubule-associated protein (Map205), and budding uninhibited by benzimidazoles 1 (BUB1). Of these three proteins, sequences targeting Plk1 from Map205 and BUB1 have not previously been explored for structure-activity relationships. Our results show that analogs of all three proteins have modest binding activity with Plk1, confirming that Map205 and BUB1 are a new source of peptide ligands. Further development of these sequences could lead to high-affinity peptidomimetic ligands that could selectively induce mitotic arrest in cancer cells.
This article has been tagged with: