Computational analysis and drug repositioning: Targeting the TDP-43 RRM using FDA-approved drugs
(1) Xi’an Middle School of Shaanxi Province, (2) Qinba State Key Laboratory of Biological Resources and Ecological Environment, College of Biological Science and Engineering, Shaanxi University of Technology, (3) Qinba State Key Laboratory of Biological Resources and Ecological Environment, College of Biological Science and Engineering, Shaanxi University of Technology / Centre of Molecular & Environmental Biology, Department of Biology, University of Minho / Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, (4) Department of Biological Sciences, Faculty of Science, National University of Singapore
https://doi.org/10.59720/23-222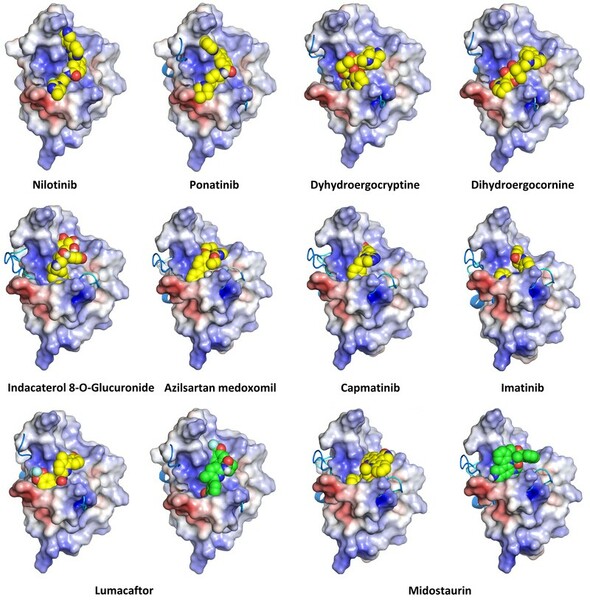
TAR DNA binding protein-43 (TDP-43) aggregation is a hallmark for many neurodegenerative diseases including amyotrophic lateral sclerosis, frontotemporal dementia and Huntington’s disease. These protein aggregates can disrupt neuronal function and contribute to neurodegeneration. Previous studies have uncovered that adenosine triphosphate (ATP) is a promising molecule to dock onto the TDP-43 RNA recognition motif (RRM) region to reduce amyloid-like aggregation. This provides a potential therapeutic strategy in which chemicals with similar binding properties could be selected as drugs. Under normal physiological conditions, TDP-43 RRM region mediates the binding of nucleic acid with TDP- 43. Therefore, we hypothesized that molecules such as tyrosine kinase inhibitors (TKIs), which can bind to ATP-binding sites or competitively bind to other nucleic acid binding regions, including different variants of RRM domains, are of great screening interest. We conducted in silico simulations using molecular dynamic simulation and virtual screening, in which the ATP-binding pocket is introduced in docking model. Our results supported our hypothesis because five of ten selected binding chemicals were TKIs. From the result, we then selected the two molecules under maximum concentration in bloodstream by conducting further screening strategies such as long-term molecular dynamic simulation, and Lipinski’s rules testing.
This article has been tagged with: