Breaking the Ice: A Scientific Take on the Ice Melting Abilities of Household Salts
(1) Princeton High School, Princeton, New Jersey, (2) Princeton University, Princeton, New Jersey
https://doi.org/10.59720/17-050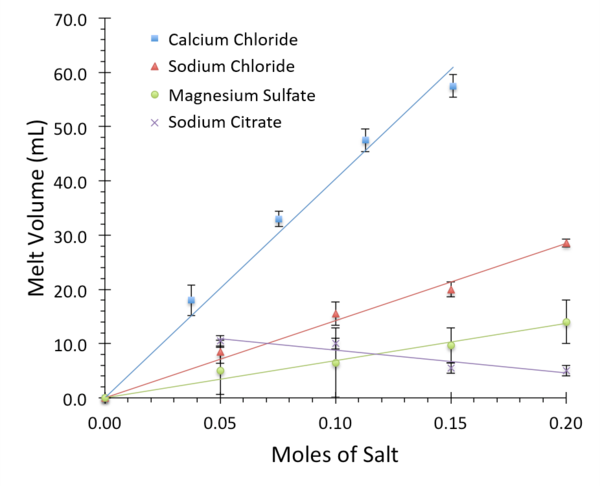
Ice is formed in a very strict hexagonal pattern, causing it to be less dense than its liquid counterpart. Dissolved salt ions disrupt the formation of this crystal lattice structure, altering the equilibrium between water and ice. This property is crucial to road safety during winter conditions, when deicing salts are used to keep roads clear. In this study, we examined the melting abilities of several subtypes of salt, including calcium chloride, sodium citrate, magnesium sulfate, and sodium chloride. These common household salts were chosen because they differ in their chemical properties, environmental impact, and physical properties – including ease of spread and cost. To quantify the effectiveness of a salt at disrupting ice structure, increasing concentrations were added to standardized ice blocks in a 4°C cold room and the volume of melt-water was recorded. The properties of freezing point depression and enthalpy of dissolution were used to form hypotheses about the chemistry of melting. Additionally, they were used to predict the melting ability of novel salt mixtures. We discovered that calcium chloride is the most effective disrupter of ice structure. This result is important because it identifies a specific salt that can be purchased for home use as the most effective melter during the winter.
This article has been tagged with: