Monitoring the formation of polyurethane foams with an infrared camera: Classroom activity
(1) Kamnoetvidya Science Academy (KVIS), Rayong 21210, Thailand, (2) Department of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology (VISTEC), Rayong 21210, Thailand
https://doi.org/10.59720/20-039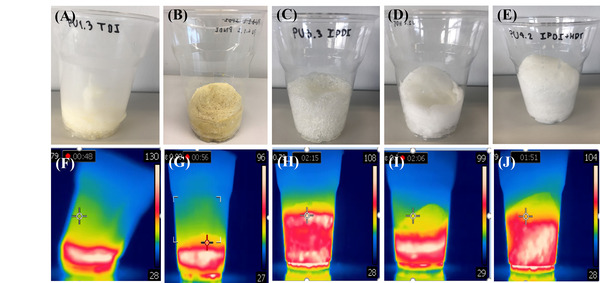
Foams prepared with polyurethanes (PU), a polymer with units in the main chain joined by urethane linkages, are commonly used in isolation panels, seals, automative seats, and bedding. The reaction causing the formation of polyurethane foams is a conventional, yet spectacular, way to attract the attention of polymer chemistry students. Infrared cameras are a relatively inexpensive apparatus that allow for semi-quantitatively temperature monitoring at the surface of objects. Because the formation of polyurethane is exothermic, the polymerization reaction and the expansion of the foam can be easily detected by an infrared camera. Information such as volume expansion, maximum temperature, temperature distribution in time and space, and reaction time can be retrieved from short videos. These data can be correlated and discussed in relationship with the reactivity of the components for synthesizing the foam. The primary goal of this activity was to investigate the exothermic reaction of polyurethane foam using an infrared camera which has been successfully used for monitoring the exothermic formation of polyurethane foams. These values can be discussed in term of reactivity of isocyanates (aromatic and aliphatic/cycloaliphatic) for a given formulation of polyols. The highly visual content of such experiments is attractive for children and students. Chemicals for performing the experiments can be donated either individually or as ready sets (“polyols”) from the industry. It is anticipated that the investment required for buying the infrared camera can be redeemed by using it for other types of exo- or endothermic reactions and for teaching heat transfer in physics class.
This article has been tagged with: