The Development of a Superhydrophobic Surface Using Electrolytic Deposition & Polymer Chains Precipitation
(1) Brooklyn Technical High School, Brooklyn, NY
https://doi.org/10.59720/20-191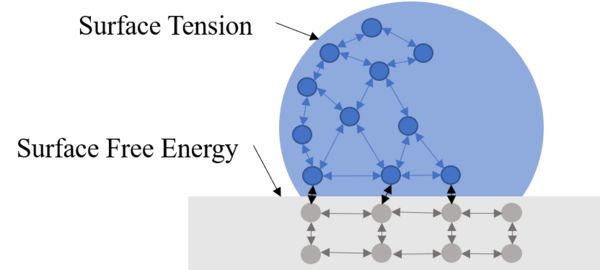
The useful life of infrastructure metals is limited by prolonged exposure to water and deposition of insoluble minerals. Advances in surface treatment suggest that both problems can be alleviated through the formation of surfaces that are hydrophobic and therefore self-cleaning. In nature, the surface of a lotus leaf displays superhydrophobicity, containing microbumps on the surface with non-polar nanofibers on the bumps. Here, we describe a process that mimics this topography. The process includes brief electrodeposition of zinc from aqueous Zn(NO3)2 followed by drying and spray-coating of a xylene silicone solution. Our results indicate that zinc coated steel has a contact angle of 130° and a sliding angle of 16°, displaying it has high hydrophobicity and self-cleaning properties. Copper yielded similar results, indicating that this method can be applied to other metals. These results suggest that a Cassie-Baxter state, the ideal droplet to surface interaction, was formed on these metal surfaces. However, further development should be done regarding the precipitation of nanofibers to maintain the created topography. Such hydrophobic surfaces would improve the longevity of metal infrastructure since its anti-rusting characteristics limits the surface’s exposure to water.
This article has been tagged with: