The Effect of Common Cations on DNA Degradation
(1) Westwood High School, Austin, Texas, (2) Klein High School, Houston, Texas, (3) Department of Integrative Biology, University of Texas at Austin, Austin, Texas
https://doi.org/10.59720/16-054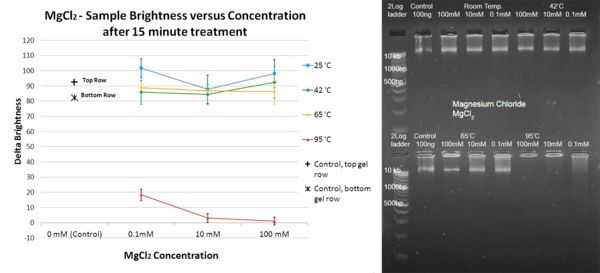
The process of DNA degradation is important to many scientific studies. Heat treatment is the standard procedure used to degrade genetic material by heating DNA-containing media past the DNA melting temperature, but certain chemical alternatives have been explored. More specifically, the presence of cations, such as Mg²⁺, has been linked to increased stability of DNA molecules subjected to high temperatures. However, the possible effects of other ions have not been extensively studied; perhaps cations similar to magnesium may offer more versatile effects. This study examines the effects that NH₄⁺, Ni²⁺, and Li⁺ have on the heat degradation and melting temperature of DNA. Magnesium chloride, magnesium sulfate, ammonium sulfate, nickel chloride, and lithium chloride solutions of different concentrations were mixed with DNA samples; the resulting mixture was heated and subsequently analyzed using gel electrophoresis. Treatment with NH₄⁺ did not yield effects that significantly differed from Mg²⁺, while Li⁺ proved effective at preserving DNA even at high temperatures. Treatment with Ni²⁺ resulted in marked degradation of DNA. These results show that magnesium, ammonium, and especially lithium ions can be used for the preservation of DNA. The specific effects of Li⁺ and Ni²⁺ on DNA are promising subjects for future research.
This article has been tagged with: